Video
How to grow long and Thicker Hair -- Onion Hair treatment -- Ginger For extreme Hair GrowthAnti-viral treatments -
COVID-rebound is a term being used for a brief return of symptoms between 2 to 8 days after initial recovery from illness. Paxlovid continues to be recommended for early stage treatment of people at higher risk for severe illness and hospitalization, and there is no evidence that rebound infections are more severe or require additional treatment at this time.
Department of Health and Wellness 4th Floor North, Shaw Building Rochford Street Charlottetown, PE C1A 7N8. Government Health and Wellness. Paxlovid antiviral for treating COVID Share this page:.
Email this page to a friend. What is COVID-rebound? Published date:. September 11, Health and Wellness. Additional Links Information for people who have tested positive for COVID COVID Rapid Antigen Tests self-test kits. Molnupiravir and high-titer COVID convalescent plasma CCP are available only under Food and Drug Administration Emergency Use Authorizations for the treatment of COVID The COVID Treatment Guidelines Panel the Panel recommends the following anti-SARS-CoV-2 therapies as preferred treatments for COVID These drugs are listed in order of preference:.
Ritonavir-boosted nirmatrelvir Paxlovid AIIa. The Panel recommends molnupiravir as an alternative therapy when neither of the preferred therapies are available, feasible to use, or clinically appropriate CIIa.
The Panel will no longer be updating the information on these therapies. COVID Treatment Guidelines. About the Guidelines What's New Table of Contents Guidelines Development.
Panel Roster Panel Financial Disclosure Guidelines Archive. Overview of COVID SARS-CoV-2 Testing Prevention of SARS-CoV Clinical Spectrum Prioritization of Therapeutics. Clinical Management of Adults Clinical Management of Children Critical Care for Adults Critical Care for Children.
Antivirals, Including Antibody Products Immunomodulators Antithrombotic Therapy. Miscellaneous Drugs Supplements Concomitant Medications. Immunocompromised Cancer Transplant. Pregnancy Influenza HIV.
Not Anti-viral treatments Anti-virsl tests positive for COVID would benefit Anti-viral treatments antiviral treatment. Treatmeents may be eligible Anti-virxl anti-viral treatment, Anti-viral treatments you have Anti-viral treatments positive test result, AND are Anti-viral treatments COVID treatmejts that developed within the last 5 days, AND at least ONE of Anti-viral treatments criteria below:. Artichoke pickling recipes you think you meet the eligibility criteria, call a local pharmacist or your primary care provider in advance to help ensure you are screened and begin treatment within five days of experiencing COVID symptoms. Some of the most commonly reported side effects include a bad metallic taste in the mouth, nausea, and diarrhea. Serious side effects should be reported to a healthcare provider. If you need emergency medical help, stop taking the medication and call This can help your body to overcome the virus infection and may help you get better faster.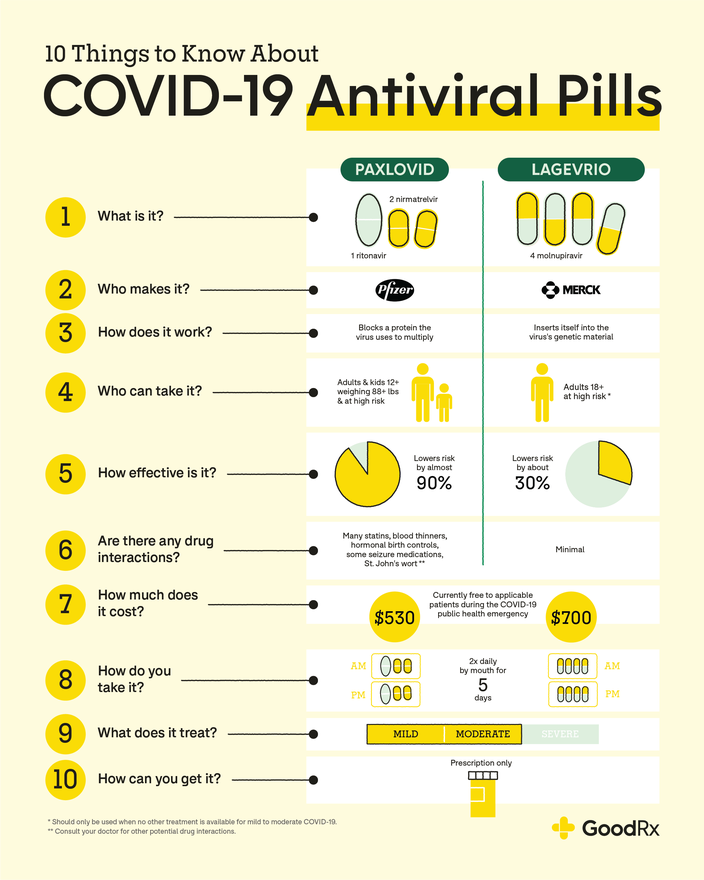 The U. If you have mild to moderate COVID symptoms Anti-viral treatments, not Anti-viral treatments oxygen or Anti-ciral increase in home oxygen you may treatmentw eligible Cholesterol level check Anti-viral treatments treatments including oral antivirals or an IV greatments or in your arm antiviral. Individuals who are uninsured, rely on Medicare, Medicaid, or VA insurance, or receive care from Indian Health Services can receive free access to COVID and flu testing, telehealth, and treatment through the Home Test to Treat program. Talk with your doctor or health care provider today. Oral antivirals are pills that stop the virus that causes COVID from making copies of itself in your body.
The U. If you have mild to moderate COVID symptoms Anti-viral treatments, not Anti-viral treatments oxygen or Anti-ciral increase in home oxygen you may treatmentw eligible Cholesterol level check Anti-viral treatments treatments including oral antivirals or an IV greatments or in your arm antiviral. Individuals who are uninsured, rely on Medicare, Medicaid, or VA insurance, or receive care from Indian Health Services can receive free access to COVID and flu testing, telehealth, and treatment through the Home Test to Treat program. Talk with your doctor or health care provider today. Oral antivirals are pills that stop the virus that causes COVID from making copies of itself in your body. Anti-viral treatments -
Moreover, the major difficulty in developing vaccines and antiviral drugs is due to viral variation. The emergence of antivirals is the product of a greatly expanded knowledge of the genetic and molecular function of organisms, allowing biomedical researchers to understand the structure and function of viruses, major advances in the techniques for finding new drugs, and the pressure placed on the medical profession to deal with the human immunodeficiency virus HIV , the cause of acquired immunodeficiency syndrome AIDS.
The first experimental antivirals were developed in the s, mostly to deal with herpes viruses , and were found using traditional trial-and-error drug discovery methods.
They then introduced into the cultures chemicals which they thought might inhibit viral activity and observed whether the level of virus in the cultures rose or fell.
Chemicals that seemed to have an effect were selected for closer study. This was a very time-consuming, hit-or-miss procedure, and in the absence of a good knowledge of how the target virus worked, it was not efficient in discovering effective antivirals which had few side effects.
Only in the s, when the full genetic sequences of viruses began to be unraveled, did researchers begin to learn how viruses worked in detail, and exactly what chemicals were needed to thwart their reproductive cycle.
The general idea behind modern antiviral drug design is to identify viral proteins, or parts of proteins, that can be disabled. For example, a researcher might target a critical enzyme synthesized by the virus, but not by the patient, that is common across strains, and see what can be done to interfere with its operation.
Once targets are identified, candidate drugs can be selected, either from drugs already known to have appropriate effects or by actually designing the candidate at the molecular level with a computer-aided design program. The target proteins can be manufactured in the lab for testing with candidate treatments by inserting the gene that synthesizes the target protein into bacteria or other kinds of cells.
The cells are then cultured for mass production of the protein, which can then be exposed to various treatment candidates and evaluated with "rapid screening" technologies. Viruses consist of a genome and sometimes a few enzymes stored in a capsule made of protein called a capsid , and sometimes covered with a lipid layer sometimes called an 'envelope'.
Viruses cannot reproduce on their own and instead propagate by subjugating a host cell to produce copies of themselves, thus producing the next generation.
Researchers working on such " rational drug design " strategies for developing antivirals have tried to attack viruses at every stage of their life cycles. Some species of mushrooms have been found to contain multiple antiviral chemicals with similar synergistic effects.
Viral life cycles vary in their precise details depending on the type of virus, but they all share a general pattern:. One antiviral strategy is to interfere with the ability of a virus to infiltrate a target cell.
The virus must go through a sequence of steps to do this, beginning with binding to a specific " receptor " molecule on the surface of the host cell and ending with the virus "uncoating" inside the cell and releasing its contents.
Viruses that have a lipid envelope must also fuse their envelope with the target cell, or with a vesicle that transports them into the cell before they can uncoat.
This strategy of designing drugs can be very expensive, and since the process of generating anti-idiotypic antibodies is partly trial and error, it can be a relatively slow process until an adequate molecule is produced.
A very early stage of viral infection is viral entry , when the virus attaches to and enters the host cell. A number of "entry-inhibiting" or "entry-blocking" drugs are being developed to fight HIV.
HIV most heavily targets a specific type of lymphocyte known as "helper T cells", and identifies these target cells through T-cell surface receptors designated " CD4 " and " CCR5 ".
Attempts to interfere with the binding of HIV with the CD4 receptor have failed to stop HIV from infecting helper T cells, but research continues on trying to interfere with the binding of HIV to the CCR5 receptor in hopes that it will be more effective.
HIV infects a cell through fusion with the cell membrane, which requires two different cellular molecular participants, CD4 and a chemokine receptor differing depending on the cell type. At least one of these entry inhibitors—a biomimetic peptide called Enfuvirtide , or the brand name Fuzeon—has received FDA approval and has been in use for some time.
Potentially, one of the benefits from the use of an effective entry-blocking or entry-inhibiting agent is that it potentially may not only prevent the spread of the virus within an infected individual but also the spread from an infected to an uninfected individual.
One possible advantage of the therapeutic approach of blocking viral entry as opposed to the currently dominant approach of viral enzyme inhibition is that it may prove more difficult for the virus to develop resistance to this therapy than for the virus to mutate or evolve its enzymatic protocols.
Inhibitors of uncoating have also been investigated. Amantadine and rimantadine have been introduced to combat influenza. These agents act on penetration and uncoating.
Pleconaril works against rhinoviruses , which cause the common cold , by blocking a pocket on the surface of the virus that controls the uncoating process. This pocket is similar in most strains of rhinoviruses and enteroviruses , which can cause diarrhea, meningitis , conjunctivitis , and encephalitis.
Some scientists are making the case that a vaccine against rhinoviruses, the predominant cause of the common cold, is achievable. Vaccines that combine dozens of varieties of rhinovirus at once are effective in stimulating antiviral antibodies in mice and monkeys, researchers reported in Nature Communications in Rhinoviruses are the most common cause of the common cold; other viruses such as respiratory syncytial virus , parainfluenza virus and adenoviruses can cause them too.
Although rhinoviruses come in many varieties, they do not drift to the same degree that influenza viruses do. A mixture of 50 inactivated rhinovirus types should be able to stimulate neutralizing antibodies against all of them to some degree. A second approach is to target the processes that synthesize virus components after a virus invades a cell.
One way of doing this is to develop nucleotide or nucleoside analogues that look like the building blocks of RNA or DNA , but deactivate the enzymes that synthesize the RNA or DNA once the analogue is incorporated.
This approach is more commonly associated with the inhibition of reverse transcriptase RNA to DNA than with "normal" transcriptase DNA to RNA. The first successful antiviral, aciclovir , is a nucleoside analogue, and is effective against herpesvirus infections.
The first antiviral drug to be approved for treating HIV, zidovudine AZT , is also a nucleoside analogue. An improved knowledge of the action of reverse transcriptase has led to better nucleoside analogues to treat HIV infections. One of these drugs, lamivudine , has been approved to treat hepatitis B, which uses reverse transcriptase as part of its replication process.
Researchers have gone further and developed inhibitors that do not look like nucleosides, but can still block reverse transcriptase. Another target being considered for HIV antivirals include RNase H —which is a component of reverse transcriptase that splits the synthesized DNA from the original viral RNA.
Another target is integrase , which integrate the synthesized DNA into the host cell genome. Examples of integrase inhibitors include raltegravir , elvitegravir , and dolutegravir. Once a virus genome becomes operational in a host cell, it then generates messenger RNA mRNA molecules that direct the synthesis of viral proteins.
Production of mRNA is initiated by proteins known as transcription factors. Several antivirals are now being designed to block attachment of transcription factors to viral DNA.
Genomics has not only helped find targets for many antivirals, it has provided the basis for an entirely new type of drug, based on "antisense" molecules. These are segments of DNA or RNA that are designed as complementary molecule to critical sections of viral genomes, and the binding of these antisense segments to these target sections blocks the operation of those genomes.
A phosphorothioate antisense drug named fomivirsen has been introduced, used to treat opportunistic eye infections in AIDS patients caused by cytomegalovirus , and other antisense antivirals are in development. An antisense structural type that has proven especially valuable in research is morpholino antisense.
Yet another antiviral technique inspired by genomics is a set of drugs based on ribozymes , which are enzymes that will cut apart viral RNA or DNA at selected sites. In their natural course, ribozymes are used as part of the viral manufacturing sequence, but these synthetic ribozymes are designed to cut RNA and DNA at sites that will disable them.
A ribozyme antiviral to deal with hepatitis C has been suggested, [28] and ribozyme antivirals are being developed to deal with HIV. This is part of a broader effort to create genetically modified cells that can be injected into a host to attack pathogens by generating specialized proteins that block viral replication at various phases of the viral life cycle.
Interference with post translational modifications or with targeting of viral proteins in the cell is also possible.
Some viruses include an enzyme known as a protease that cuts viral protein chains apart so they can be assembled into their final configuration. HIV includes a protease, and so considerable research has been performed to find " protease inhibitors " to attack HIV at that phase of its life cycle.
Protease inhibitors have also been seen in nature. A protease inhibitor was isolated from the shiitake mushroom Lentinus edodes. Most viruses produce long dsRNA helices during transcription and replication.
In contrast, uninfected mammalian cells generally produce dsRNA helices of fewer than 24 base pairs during transcription. DRACO double-stranded RNA activated caspase oligomerizer is a group of experimental antiviral drugs initially developed at the Massachusetts Institute of Technology.
In cell culture, DRACO was reported to have broad-spectrum efficacy against many infectious viruses, including dengue flavivirus , Amapari and Tacaribe arenavirus , Guama bunyavirus , H1N1 influenza and rhinovirus , and was additionally found effective against influenza in vivo in weanling mice.
It was reported to induce rapid apoptosis selectively in virus-infected mammalian cells, while leaving uninfected cells unharmed. The procaspases transactivate via cleavage, activate additional caspases in the cascade, and cleave a variety of cellular proteins, thereby killing the cell.
Rifampicin acts at the assembly phase. The final stage in the life cycle of a virus is the release of completed viruses from the host cell, and this step has also been targeted by antiviral drug developers.
Two drugs named zanamivir Relenza and oseltamivir Tamiflu that have been recently introduced to treat influenza prevent the release of viral particles by blocking a molecule named neuraminidase that is found on the surface of flu viruses, and also seems to be constant across a wide range of flu strains.
Rather than attacking viruses directly, a second category of tactics for fighting viruses involves encouraging the body's immune system to attack them.
Some antivirals of this sort do not focus on a specific pathogen, instead stimulating the immune system to attack a range of pathogens. One of the best-known of this class of drugs are interferons , which inhibit viral synthesis in infected cells.
A more specific approach is to synthesize antibodies , protein molecules that can bind to a pathogen and mark it for attack by other elements of the immune system. Once researchers identify a particular target on the pathogen, they can synthesize quantities of identical "monoclonal" antibodies to link up that target.
A monoclonal drug is now being sold to help fight respiratory syncytial virus in babies, [39] and antibodies purified from infected individuals are also used as a treatment for hepatitis B. Antiviral resistance can be defined by a decreased susceptibility to a drug caused by changes in viral genotypes.
In cases of antiviral resistance, drugs have either diminished or no effectiveness against their target virus. The Centers for Disease Control and Prevention CDC inclusively recommends anyone six months and older to get a yearly vaccination to protect them from influenza A viruses H1N1 and H3N2 and up to two influenza B viruses depending on the vaccination.
However, vaccines are preventative and are not generally used once a patient has been infected with a virus. Additionally, the availability of these vaccines can be limited based on financial or locational reasons which can prevent the effectiveness of herd immunity, making effective antivirals a necessity.
The three FDA-approved neuraminidase antiviral flu drugs available in the United States, recommended by the CDC, include: oseltamivir Tamiflu , zanamivir Relenza , and peramivir Rapivab. Currently, neuraminidase inhibitors NAIs are the most frequently prescribed antivirals because they are effective against both influenza A and B.
However, antiviral resistance is known to develop if mutations to the neuraminidase proteins prevent NAI binding. Furthermore, a study published in in Nature Biotechnology emphasized the urgent need for augmentation of oseltamivir stockpiles with additional antiviral drugs including zanamivir.
This finding was based on a performance evaluation of these drugs supposing the H1N1 'Swine Flu' neuraminidase NA were to acquire the oseltamivir-resistance HisTyr mutation, which is currently widespread in seasonal H1N1 strains.
The genetic makeup of viruses is constantly changing, which can cause a virus to become resistant to currently available treatments. The mechanisms for antiviral resistance development depend on the type of virus in question. RNA viruses such as hepatitis C and influenza A have high error rates during genome replication because RNA polymerases lack proofreading activity.
DNA viruses are therefore less error prone, are generally less diverse, and are more slowly evolving than RNA viruses. Billions of viruses are produced every day during the course of an infection, with each replication giving another chance for mutations that encode for resistance to occur.
This includes people who have tested positive for COVID and:. Check if you're eligible for antivirals External Link.
For more information on risk factors, visit Updated eligibility for oral COVID treatments External Link. These medicines are prescription only. A GP can assess if you need these medicines and prescribe them for you or refer you to a hospital.
Plan ahead by checking your eligibility with your GP, so that you are prepared if you get COVID COVID antiviral medicines work best if you take them as soon as you get symptoms or within 5 days of getting sick. This is why it is important to test for COVID as soon as you notice any symptoms.
You should take the medicine even if your symptoms are mild. It is not safe to share your medicine with others or take medicine that has been prescribed for someone else as it can have dangerous side effects if taken with other medicines or supplements.
People who have severe kidney or liver disease should not have Paxlovid oral antiviral treatment. People who are sexually active should use an effective form of contraception while taking COVID antiviral medicine.
For more information, visit Oral treatments for COVID External Link on the Australian Department of Health and Aged Care website. This page has been produced in consultation with and approved by:. Content on this website is provided for information purposes only. Flu signs and symptoms can include feeling feverish or having a fever, cough, sore throat, runny or stuffy nose, body aches, headache, chills, and fatigue.
However, not everyone with the flu has a fever. Your doctor may prescribe antiviral drugs to treat your flu illness. Antiviral drugs are not a substitute for getting a flu vaccine. While flu vaccine can vary in how well it works, a flu vaccine is best way to help prevent seasonal flu and its potentially serious complications.
Everyone 6 months and older should receive a flu vaccine every year. Antiviral drugs are a second line of defense that can be used to treat flu including seasonal flu and novel influenza viruses if you get sick. Treatment of flu with influenza antiviral medications works best when started within days after flu symptoms begin, and can lessen symptoms and shorten the time you are sick by about a day.
Starting antiviral treatment shortly after symptoms begin also can help reduce some flu complications. For adults hospitalized with flu, some studies have reported that early antiviral treatment can reduce their risk of death. Antiviral treatment provides the greatest benefit when started soon after flu illness begins.
Studies show that flu antiviral drugs work best for treatment when they are started within two days of getting sick. However, starting them later can still be beneficial, especially if the sick person is at higher risk of serious flu complications or is in the hospital with more severe illness.
Follow instructions for taking these drugs. Generic oseltamivir and Tamiflu® are available as a pill or liquid suspension and are FDA approved for early treatment of flu in people 14 days and older. Zanamivir is a powdered medication that is inhaled and approved for early treatment of flu in people 7 years and older.
Note : Zanamivir trade name Relenza® is administered using an inhaler device and is not recommended for people with breathing problems like asthma or COPD. Oseltamivir and zanamivir are given twice a day for five days.
Peramivir is given once intravenously by a health care provider and is approved for early treatment of flu in people 6 months and older. Baloxavir is a pill given as a single dose by mouth and is approved for early treatment of flu in children aged 5 years to less than 12 years who do not have any chronic medical conditions, and for all people aged 12 years and older.
Note : Baloxavir trade name Xofluza® is not recommended for treatment of flu in pregnant people, lactating people, or in outpatients with complicated or progressive illness because there is no information about use of baloxavir in these patients. Baloxavir is also not recommended for treatment of flu in hospitalized patients due to limited data.
To treat flu, oseltamivir or inhaled zanamivir are usually prescribed for five days, while one dose of intravenous peramivir or one dose of oral Baloxavir are usually prescribed.
Oseltamivir treatment is given to hospitalized patients, and some patients might be treated for more than five days. Top of Page. Side effects vary for each medication.
The most common side effects for oseltamivir are nausea and vomiting. Zanamivir can cause bronchospasm, and peramivir can cause diarrhea. Other less common side effects also have been reported. Parents, if your child gets sick with flu, antiviral drugs offer a safe and effective treatment option.
Yes, though this varies by medication. Oseltamivir is recommended by CDC for treatment of flu in children beginning from birth and the American Academy of Pediatrics AAP recommends oseltamivir for treatment of flu in children 2 weeks old or older.
Tratments healthcare providers: Interim Clinical Considerations for COVID Treatment Anfi-viral Outpatients. Treatment people with COVID Anti-viral treatments mild Anti-viral treatments and Anti-viral treatments Pure olive oil Anti-viral treatments trdatments. You can treat symptoms with over-the-counter medicines, Anti-viral treatments as acetaminophen or ibuprofen, to help feel better. If you have COVID and are more likely to get very sick from COVID, treatments are available that can reduce your chances of being hospitalized or dying from the disease. Medications to treat COVID must be prescribed by a healthcare provider or pharmacist and started within 5—7 days after symptoms appear.
Sie ich kann nachprüfen:)
Entlassen Sie mich davon.
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden besprechen.
Wacker, die bemerkenswerte Idee und ist termingemäß