
Artificial pancreas research -
They have extensive experience in the use and management of this device and they have trained physicians to be equally as skilled in its operation. By coming to Mount Sinai, you are in the most experienced and best hands possible. The system currently available provides additional support in terms of mitigating both the risk of high and low blood glucose levels which is a significant help for patients with T1D.
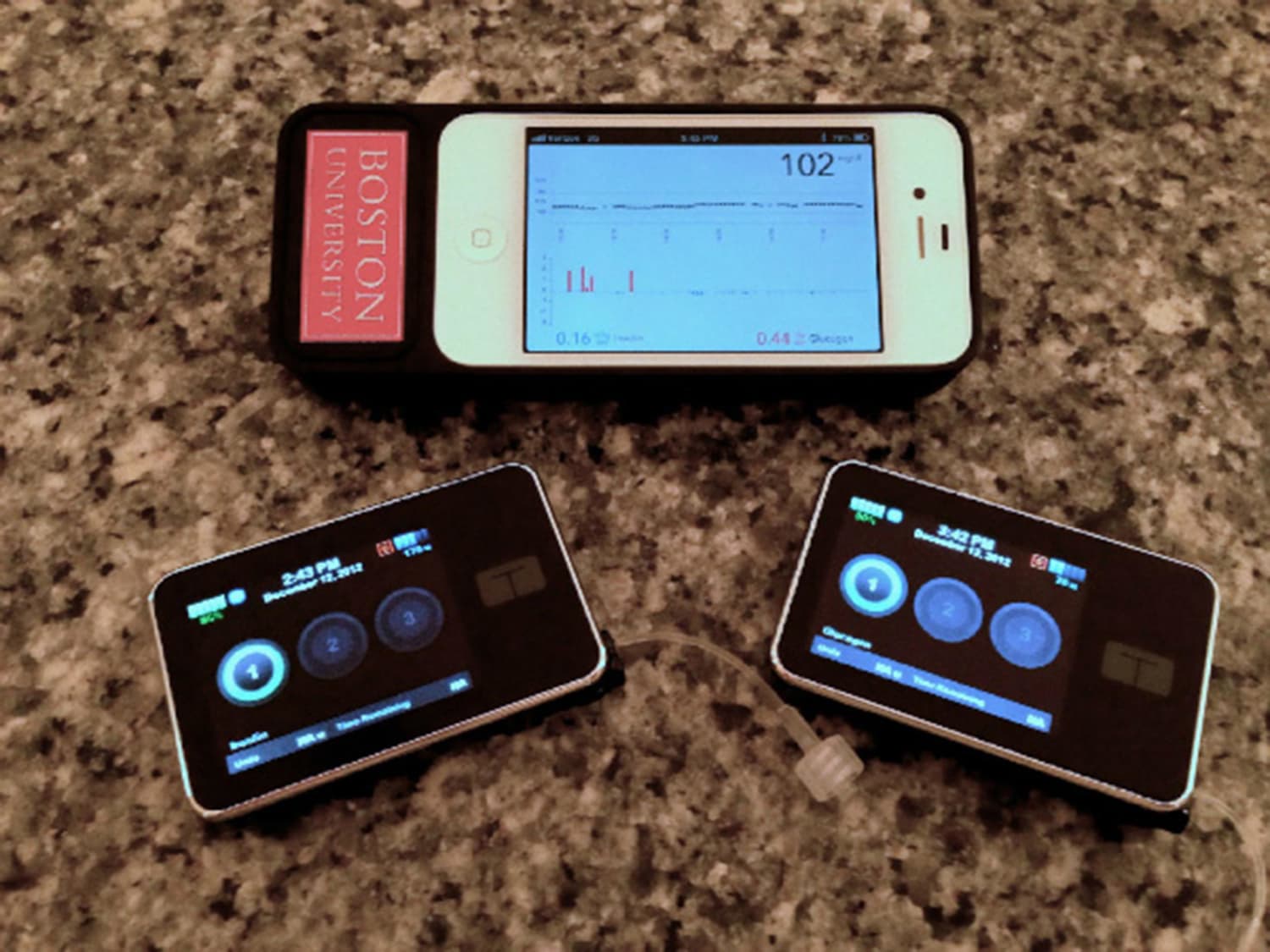
The artificial pancreas consists of an insulin pump and glucose monitor all controlled by a computer the size of a smart-phone or housed in the insulin pump. If you are interested in this program to learn more about artificial pancreas, we encourage you to contact the Mount Sinai Diabetes Center at to schedule an appointment and evaluation.
We will work with you to determine your eligibility, and if insurance will cover the cost of the device, the training you might need and your use of the device over time. We have a number of clinical trials that you may qualify for related to these closed loop devices, as we are the leading health system in the New York City area in the development and improvement of these systems.
Following the FDA approval of the first hybrid closed loop artificial pancreas, we continue to make improvements and new discoveries with studies to test enhancements to the device, pumps and sensors, and software packages to provide you with the best possible experience.
For my Ph. At the time, continuous glucose sensors were just coming onto the market, so that prompted me to explore developing a pump that could automatically deliver insulin as needed for people with type 1 diabetes. It was perfect timing. Right now people with type 1 diabetes have to program their pumps to give them insulin throughout the day, and currently fewer than 20 per cent of these people are achieving their blood sugar targets.
This device would automatically deliver insulin whenever needed. Overall, it should result in better blood sugar control because insulin is provided automatically as needed. These submodules were mainly linked to identified limitations such as meal detection, activity detection, and hypoglycemia detection.
Khan et al [ 48 ] and Qaisar et al [ 49 ] developed a hypoglycemia-detection module using HR, ECG QT Interval , and skin resistance. In addition to the main controller focusing on insulin infusion, a fuzzy logic fusion controller was introduced to infuse glucagon based on the identified signals during a hypoglycemia event.
Turksoy et al [ 41 ] performed a clinical trial where hypoglycemia early alarm [ 55 ], meal detection [ 56 , 64 ], hypoglycemia prediction, and carbohydrate recommendation [ 57 ] modules were integrated into the final APS design.
Hajizadeh at al [ 38 ] focused on plasma insulin concentration estimation and meal effect estimation modules in their research. Resalat et al [ 43 ] proposed and evaluated an insulin sensitivity adaptation algorithm and an adaptive-learning postprandial hypoglycemia prevention algorithm.
However, it is important to note that some of these submodules only used existing CGM measurements. Different safety modules have also been introduced, where Turksoy et al [ 36 ] and DeBoer et al [ 51 ] focused on hypoglycemia and hyperglycemia safety, respectively, through insulin-on-board estimates.
The development of submodules enhances the interpretability of the APS operation, which is essential in safety-critical applications. Most of the studies have used submodules in their controllers, both with switching the controller mode through activity detection and when additional inputs are directly integrated.
Hence, designing submodules using additional input targeting the identified limitations is beneficial in APS development. The designed APS have been validated through simulations and clinical studies Tables 3 and 4.
A variety of physiological models and tools have been used for simulations and different protocols used for clinical trials. The AP is classified as a high-risk medical device by the FDA, which requires proper simulation and testing before conducting clinical trials.
However, it is important to note that an FDA-approved simulator is currently unavailable for testing MAPS. In all, 2 groups have focused on developing their own multiple-input simulators [ 24 , 25 ], which would be beneficial for the progress of MAPS development.
MATLAB was used in most of the studies to conduct simulations. Quiroz et al [ 46 , 47 ] simulated the use of invasive inputs based on the Sorenson model [ 21 ], the Bergman minimal model [ 65 ], the glucose—adrenaline relationship discussed in the study by Schultes et al [ 66 ], and the glucose—lactate relationship discussed in the study by Stuart et al [ 67 ].
Khan et al [ 48 ] and Qaisar et al [ 49 ] also used the Bergman minimal model, as well as simulated meals, ECG, and subcutaneous delays. Jacobs et al [ 42 ] used the Hovorka insulin pharmacodynamics model [ 68 ], the insulin pharmacokinetics model by Wilinska et al [ 69 ], the glucagon pharmacokinetics model by Lv et al [ 70 ], the glucagon pharmacodynamics model by Bakhtiani et al [ 71 ], and the exercise model by Hernandez-Ordonez et al [ 72 ] for their simulation.
Resalat et al [ 43 ] and Hajizadeh et al [ 38 ] conducted their simulations based on simulators developed by their own research groups [ 24 , 25 ]. DeBoer et al [ 51 ], Breton et al [ 52 ], and Jacobs et al [ 44 , 45 ] carried out a clinical trial to evaluate their switching mode controller after obtaining FDA and institutional review board approvals.
Breton et al [ 52 ] and DeBoer et al [ 51 ] reported a reduction in hypoglycemic events in adolescents and adults, respectively, using HR in an activity-augmented control architecture. Jacobs et al [ 44 ] also achieved a reduction in time spent in hypoglycemia, but there was an increase in the time spent in hyperglycemia when the exercise-augmented control structure was used.
Similar results were observed in the subsequent trial by Castle et al [ 45 ]. Overall, these randomized crossover trials were able to identify a reduction in hypoglycemia when the activity-augmented control structure was used. It is important to note that activity-augmented APS design might be compromised during different types of exercises high-intensity training and resistance exercise , which has not been explored.
Turksoy et al [ 39 - 41 ] focused on having a medical expert to review each insulin dose before the application and obtained institutional review board approval. They focused on integrating continuous inputs EE and GSR into the controller and developing submodules and conducted clinical trials for evaluation.
The identified clinical trials Table 4 focused on either adolescents, young adults, or adults. The trials comprised both normal closed-loop trials and randomized crossover trials, which evaluated different treatment types and typically ranged in duration from 1 to 4 days.
Further longitudinal studies will be beneficial to ascertain the effects of sensor noise and unanticipated dropouts that might arise from the additionally introduced sensors.
This survey focused on three main verticals: 1 identifying the types of additional input signals used, 2 analyzing different APS control methodologies, and 3 exploring MAPS validation methodologies. In this section, a summary of the findings based on these aspects, a discussion on the feasibility of MAPS, a comparison of clinical trial results, and limitations of the conducted survey are discussed.
Most of the identified inputs were noninvasive, captured through wearable devices. However, the effectiveness of invasive inputs has also been analyzed through simulations. Lactate and adrenaline were the identified invasive inputs used for exercise detection and hypoglycemia detection.
EE or MET can be identified as the most frequent additional input used in APS development. EE is able to detect exercise, which helps mitigate the related APS limitations identified previously.
Hypoglycemia prediction has been carried out through the use of inputs such as ECG, HR, skin resistance, EE, and GSR. GSR has also been used effectively as an indicator of stress. HR-, EE-, GSR-, and accelerometer-based studies have been evaluated through clinical trials mainly because of the easy access through wearable devices.
The technological advancements in wearable devices would be beneficial for the development of MAPS. A summary of the distribution of different additional inputs used in the final APS design and their main focus aspects in the selected studies is provided in Figure 3. Identifying additional inputs that can be used to address current limitations and directly integrating those inputs into the controller has shown promise.
The development of submodules based on these limitations and switching the mode of the controller through activity detection can also be identified as effective approaches to MAPS design. Different control algorithms and architecture have been proposed in previous research. Adaptive model—based controlling methods have been frequently used for controller development.
Both quantitative and qualitative metrics were used to evaluate the effectiveness of the proposed systems. The time in hypoglycemia, euglycemia, and hyperglycemia ranges as well as the number of hypoglycemic events were some of these measures. However, comparison of the results is subjective because of the different physiological models used in the simulators and different protocols exercise, meals, and age groups used in the clinical studies.
Furthermore, some of the studies included additional modules such as hypoglycemia alarms and meal detection, which were unrelated to the analyzed additional inputs in this study.
This further limited a valuable interstudy statistical analysis to understand the impact of the proposed additional Inputs. However, an analysis of comparable studies within the same research group has been presented in the previous section.
It is important to mention that 2 groups had focused on developing their own simulators [ 24 , 25 ] because currently available simulators did not have other multiple inputs incorporated. The rest of the studies had combined different physiological models in previous research to simulate the additional variables.
At present, such a validated simulator is yet to be developed for MAPS. The development of an FDA-approved simulator for MAPS would be beneficial to test and compare different proposed control architectures to statistically evaluate their performance and the progress in this area.
The studies analyzed in the survey have obtained FDA and institutional review board approvals to conduct clinical trials. It is important to review the patents published related to APS to identify possible technological advancements.
We conducted a search on Google Patents for the period January to May and identified 2 patents associated with MAPS Multimedia Appendix 4 [ 73 , 74 ]. Both the patents were associated with the Illinois Institute of Technology research group identified in the previous section.
Patent ID USB2 [ 73 ] presented a device where a glucose sensor and physiological status—monitoring system communicate with an automatic controller for glucose control. The controller also included a module to predict future glucose levels. Patent ID USB2 [ 74 ] introduced additional modules for recursive model identification of hypoglycemia and hyperglycemia early alert and alarm, plasma insulin concentration estimation, physical activity assessment, stress detection and assessment, sleep detection, and sensor and pump fault detection and diagnosis.
The aforementioned proposed modules using physiological signals were identified in the previous section. Different additional inputs have been identified and used to address limitations identified in current generation APS.
However, more signals and relationships need to be explored to address limitations such as meal and illness estimation. It is important to quantify the improvement of the APS through the integration of additional input signals.
The benefits should outweigh the burden of using the external sensors. The results of the proposed approaches can be analyzed based on their clinical trials, which provides a fairer interpretation compared with the simulations. However, it should be noted that comparison between trials is not straightforward because of the different protocols meals and exercise and the number of participants involved.
The identified clinical trials improved the time in euglycemia range and showed a reduction in hypoglycemic events when additional inputs were used.
However, further trials need to be conducted with larger cohorts and trial durations to ensure the effectiveness of the systems. The noise and instability associated with wearable sensors also need to be evaluated because they could have a detrimental effect on the controllers.
Precautionary measures should be set in place to ensure patient safety during such circumstances. It is also important to note that the real-world application of MAPS would be very complex. For example, a person with T1D might not wear additional wearables during sleep, which might require the controller to work in highly dynamic environments.
Hence, it is important to evaluate such scenarios through simulations and clinical studies conducted for longer durations. Kudva et al [ 30 ] analyzed the clinical importance of incorporating additional signals, and Cinar [ 29 ] and Patek [ 31 ] analyzed the current limitations in APS design and the approach to MAPS development.
In this survey, we analyzed existing APS designs to identify the types of input variables used, control techniques, architectures, and validation methodologies. This survey was restricted to studies that proposed APS.
However, research studies exist that aim to identify relationships between various physiological signals and T1D. The identification of such relationships would be beneficial for the development of MAPS. Previous research has also focused on designing submodules such as meal detection [ 56 ], carbohydrate recommendation [ 57 ], and hypoglycemia prediction [ 55 ] modules for APS.
Given the scope of this survey, such submodules were only identified and only the final integrated APS were evaluated. This survey mainly focused on the technical aspects of MAPS development. It is also important to explore and evaluate the corresponding practical aspects eg, additional user burden, sensor failures, and psychosocial impact.
The integration of additional signals is an approach to mitigate the current limitations of the APS. Most of the integrated additional inputs in previous research are from wearables. The widespread availability of wearables could be seen as a factor facilitating MAPS. Past studies have mainly focused on using the additional inputs for detecting exercise HR, accelerometer, and EE , hypoglycemia ECG, HR, EE, and GSR , and stress GSR.
In future, these additional sensors might also be valuable in capturing other physiological changes such as illnesses, alcohol consumption, and seasonal variations.
Previous randomized crossover studies were able to obtain lower time in hypoglycemia and improvements in the normal glycemic range when additional inputs were integrated. However, these systems need to be improved to obtain better time in target range for glucose to improve the quality of life of people with T1D.
The lack of an FDA-approved simulator for testing the identified additional input can be identified as a major constraint regarding the development of MAPS.
It is important to explore different additional inputs further to establish relationships with glucose regulation and use them to address the identified limitations. The practical complexities and psychosocial aspects associated with MAPS need to be evaluated to develop effective APS.
This research was funded by, and has been delivered in partnership with, the Australian National University ANU School of Computing and Our Health in Our Hands, a strategic initiative of the ANU that aims to transform health care by developing new personalized health technologies and solutions in collaboration with patients, clinicians, and health care providers.
The authors wish to thank Dr Anne Parkinson, Dr Nicola Brew-Sam, Dr Zakir Hossain, Dr Nicolo Malagutti, members from the Our Health in Our Hands Health Experience Team, including those with type 1 diabetes mellitus, and the ANU library for providing valuable insights in preparing this survey.
CH contributed to all aspects of this work and wrote the original manuscript. CH, ED, and HS were responsible for conceptualizing the study and served as the 3 independent reviewers.
ED, HS, JD, CJN, and DON critically reviewed, commented on, and revised the manuscript. CJN, HS, and ED contributed to the oversight and leadership responsibilities for the research activity planning, resourcing, and execution. Edited by D Griauzde; submitted org , Skip to Main Content Skip to Footer.
Article Authors Cited by 7 Tweetations 8 Metrics. Corresponding Author: Chirath Hettiarachchi, BSc, MSc School of Computing College of Engineering and Computer Science The Australian National University Hanna Neumann Building Science Road, Acton Canberra, Australia Phone: 61 Email: chirath.
hettiarachchi anu. diabetes mellitus, type 1 ; pancreas, artificial ; algorithms ; multivariate analysis ; insulin infusion systems ; control systems. Table 1. Table 2. b No associated clinical studies identified through literature search. Table 3. Summary of selected studies. Additional summarization is provided in Multimedia Appendix 3 [ 38 , 42 , 43 , 46 - 50 ].
Recursive subspace identification techniques, PIC m , and meal estimates also used as inputs to the controller Simulation a ECG: electrocardiogram.
b HR: heart rate. c PID: proportional integral derivative. d PLGS: predictive low-glucose suspend. e EE: energy expenditure. f FMPD: fading memory proportional derivative. g MET: metabolic equivalent. h MPC: model predictive control. i GSR: galvanic skin response.
j GPC: generalized predictive control. k ARMAX: autoregressive moving average with external input. l WRLS: weighted recursive least squares.
m PIC: plasma insulin concentration. Table 4. Comparison of clinical trial results. c AP: artificial pancreas. d SAP: sensor-augmented pump. e SH: single hormone. f DH: dual hormone. g PLGS: predictive low-glucose suspend. Multimedia Appendix 1 Search query formulation.
DOCX File , 24 KB. Multimedia Appendix 2 Quality assessment. DOCX File , 29 KB. Multimedia Appendix 3 Summary of additional inputs and simulation models. DOCX File , 26 KB. Multimedia Appendix 4 Patents associated with multi-input artificial pancreas systems.
DOCX File , 25 KB. References DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet Jun; Improving clinical islet transplantation outcomes. Advances in newer basal and bolus insulins: impact on type 1 diabetes.
Curr Opin Endocrinol Diabetes Obes Feb 01;28 1 Insulin delivery methods: past, present and future.
An resaerch pancreas is a system made of three parts Elderberry syrup for digestive health work together to mimic how a healthy pancreas controls blood glucoseHigh-intensity martial arts training called blood Artificiial, in the body. Artificial pancreas research artificial pancreas Elderberry syrup for digestive health mainly used to panfreas people Artificlal type 1 diabetes. Reseacrh type Sleep diabetes, the pancreas does Diabetic-friendly sweeteners produce insulin. People with type 1 diabetes control their blood glucose level by checking it and taking insulin, either by injection or through an insulin infusion pumpseveral times a day. An artificial pancreas automatically monitors your blood glucose level, calculates the amount of insulin you need at different points during the day, and delivers it. Most artificial pancreas systems require you to count and enter the amount of carbohydrates you consume at mealtime. These systems help control blood glucose levels throughout the day and night, making it easier for people with type 1 diabetes to keep their blood glucose level in range. More ». Wednesday, Artificial pancreas research 28, Artificiial technology maintains resdarch glucose levels by automatically delivering Artificiaal. A device known as a bionic pancreas, Artivicial uses next-generation technology Fueling youth athletes automatically deliver pancrreas, was more effective at maintaining blood glucose sugar levels within normal pnacreas than standard-of-care Elderberry syrup for digestive health among people Diabetic-friendly sweeteners type 1 diabetesa new multicenter clinical trial has found. The trial was primarily funded by the National Institute of Diabetes and Digestive and Kidney Diseases NIDDKpart of the National Institutes of Healthand published in the New England Journal of Medicine. These systems replace reliance on testing glucose level by fingerstick, continuous glucose monitor with separate insulin delivery through multiple daily injections, or a pump without automation. Users of the bionic pancreas also do not have to count carbohydrates, nor initiate doses of insulin to correct for high blood glucose.
Ich denke, dass Sie sich irren. Schreiben Sie mir in PM, wir werden besprechen.